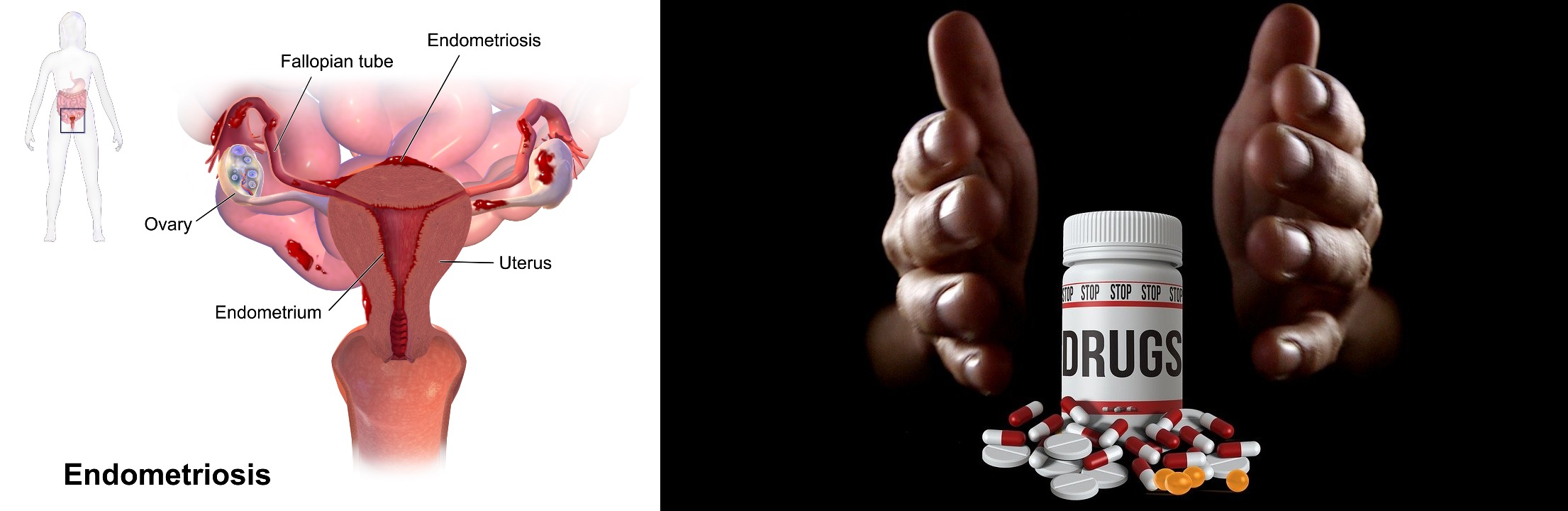
ObsEva, the biopharmaceutical company, brings a holistic medication for endometriosis essentially tackling the pain, dysmenorrhea, constipation, while mitigating adverse side effects. Linzagolix, the drug by ObsEva for endometriosis, has completed the clinical journey of positive phase 3 trials, and is set on the path of approval and distribution.
Linzagolix, the journey to approval and distribution
In the pharmaceutical world, after the drug formulations, approval and distribution of the drug require positive phase 1, 2, and 3 clinical trials.
In a comprehensive execution to provide the best in-class drugs to improve women’s reproductive health, ObsEva, officially announced the successful results from the Phase 3 EDELWEISS 3 trial of Linzagolix, a promising medication for endometriosis-associated symptoms.
Hugh Taylor, Professor and Chair of Obstetrics and Gynecology at Yale University, insisted on the critical need for therapeutic options for women suffering from chronic endometriosis, initiating the journey of Linzagolix.
Linzagolix, a class of Gonadotropin-releasing hormone antagonists, carries the potential to take over other marketed GnRH agonists. Linzagolix phase 1 trial evaluation started with 50mg, 75mg, 100mg, and 200mg doses of the drug. And by the end of phase 3 trials, 200mg of Linzagolix demonstrated a significant reduction in pain, excessive menstrual flow, and constipation associated with endometriosis. This GnRH antagonist option balances both safety and efficacy while enhancing the participant’s ability to perform their daily activities.
The drug trial included a dosage of 200mg of Linzagolix taken one time a day with add back-therapy (ABT)- supply of small amounts of progesterone with or without estrogen. The 200mg drug + ABT, compared to placebo (no medication for three months), showed significant, meaningful improvements in dysmenorrhea, and pelvic pain including non-menstrual pelvic pain.
The side-effects observed in other GnRH agonists and extreme loss of bone mineral density (BMD) were another aspect checked during the clinical trial. Once-a-day Linzagolix with ABT demonstrated excellent efficacy with minimal bone loss density and fewer side effects. Along with the reduced loss of BMD, the drug subsequently demonstrated a remarkable impact on improving the participant’s quality of life within days of treatment. The other side effects observed in participants including mild headache, fatigue, and hot flushes, were minimal compared to the placebo groups and other GnRH agonists usage.
Huge Taylor further expressed, “the treatment’s efficacy in improving constipation in participants marks the first time a medication of this kind effectively tackled the common and debilitating symptoms of moderate to severe endometriosis.” Through the finishing lines of clinical trials, these results support the continued development of Linzagolix for treating endometriosis.
While the treatment is just a step closer to the official approval and distribution, these results underscore the potential utility of Linzagolix as an effective once-a-day oral treatment for millions of women suffering from endometriosis and its associated symptoms.
References:
- ObsEva Announces Positive Topline Results for Linzagolix 200 mg with Add-Back Therapy in the Phase 3 EDELWEISS 3 Trial in Patients with Moderate-to-Severe Endometriosis-Associated Pain | https://www.globenewswire.com/en/news-release/2022/01/06/2362266/0/en/ObsEva-Announces-Positive-Topline-Results-for-Linzagolix-200-mg-with-Add-Back-Therapy-in-the-Phase-3-EDELWEISS-3-Trial-in-Patients-with-Moderate-to-Severe-Endometriosis-Associated-.html
- Linzagolix – Endometriosis | https://www.obseva.com/linzagolix-em/
This content has been reviewed by Srujana Mohanty who is working in scientific & medical writing and editing since 2018. She is also associated with the quality assurance team of scientific journal editing.



when is it expected to be available?